What is a Solid Oxide Fuel Cell ?
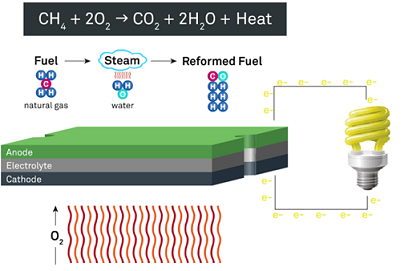
A solid oxide fuel cell consists of three parts: an electrolyte, an anode, and a cathode.
The electrolyte is a solid ceramic material, and the anode and cathode are made from special powders that sandwiched the electrolyte. Unlike other types of fuel cells, no precious metals, corrosive acids, or molten materials are required. Next, an electrochemical reaction converts fuel and air into energy, specifically electricity and heat without combustion.
Solid Oxide Fuel Cell (SOFC) works under a high temperature. At high temperature (always about 750~850℃), warmed air enters the cathode side of the fuel cell and steam reformed fuel enters to the anode side.
Next, the electrochemical reaction occurs in the electrolyte. As the reformed fuel crosses the anode, it attracts oxygen ions from the cathode. The oxygen ions combine with the reformed fuel to produce electricity, water, and small amounts of carbon dioxide. The process also generates the heat required by the fuel cell.
As long as there's fuel, air, and heat, the process continues producing clean, reliable, affordable energy.

